The clonoSEQ® Report
Disease burden at your fingertips
MRD monitoring with clonoSEQ involves 2 testing steps, with the results provided on separate reports
First report:
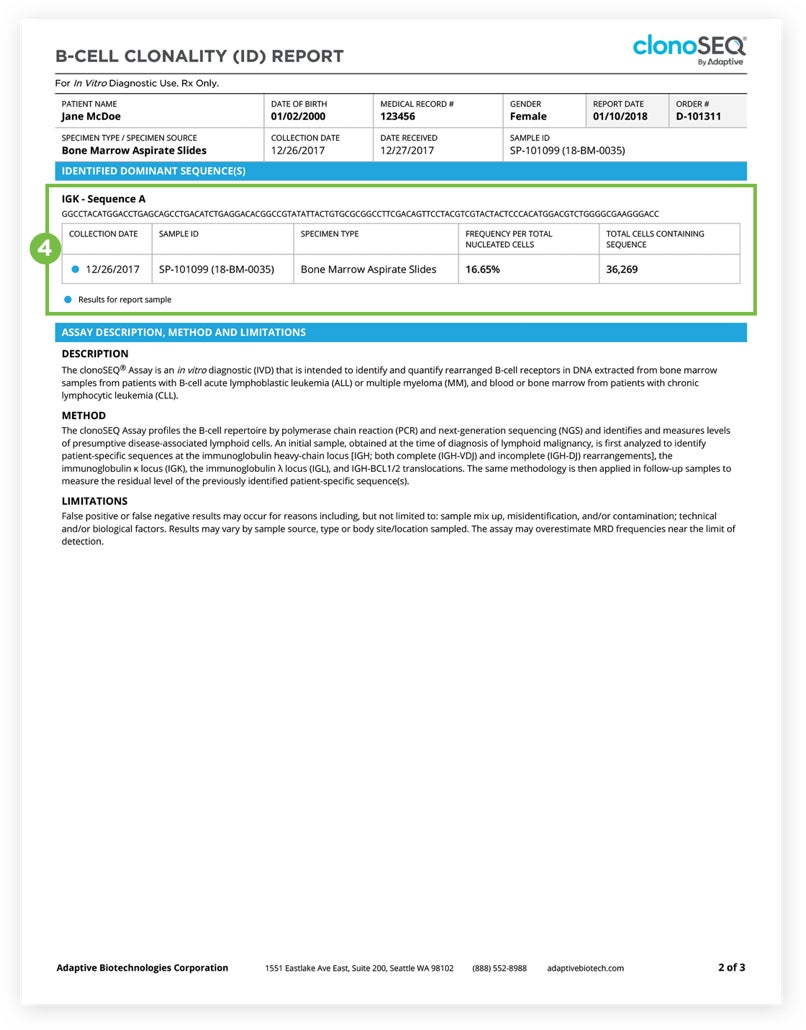
Clonality (ID) report
The Clonality (ID) Report—the first report that a clinician receives upon initiating clonoSEQ testing—provides an overview of the dominant DNA sequences identified in a patient sample collected during diagnostic workup. These dominant sequences are typically associated with malignancy and will be used by clonoSEQ as the basis for MRD detection.1
Example Clonality Sequence:
DOMINANT DNA SEQUENCE(S) IDENTIFIED
Specific to your patient’s cancer
AATACGATACGCATTTCAGCATCGCAT
CGATACGCATTTCAGCATCGCATAATA
Present in 71% of total nucleated cells
TTCGACTCAGACGCGAATCAGCATCA
ACTCAGACGCGAATCAGCATCATTCG
Present in 70% of total nucleated cells
Tap the numbers on the report for more information.
IGHV mutation status results* are also appended to the Clonality ID report for CLL patients.
*IGHV testing is available as a CLIA-validated LDT and has not been cleared or approved by the FDA.
Start ordering clonoSEQ in routine clinical practice in a few simple steps
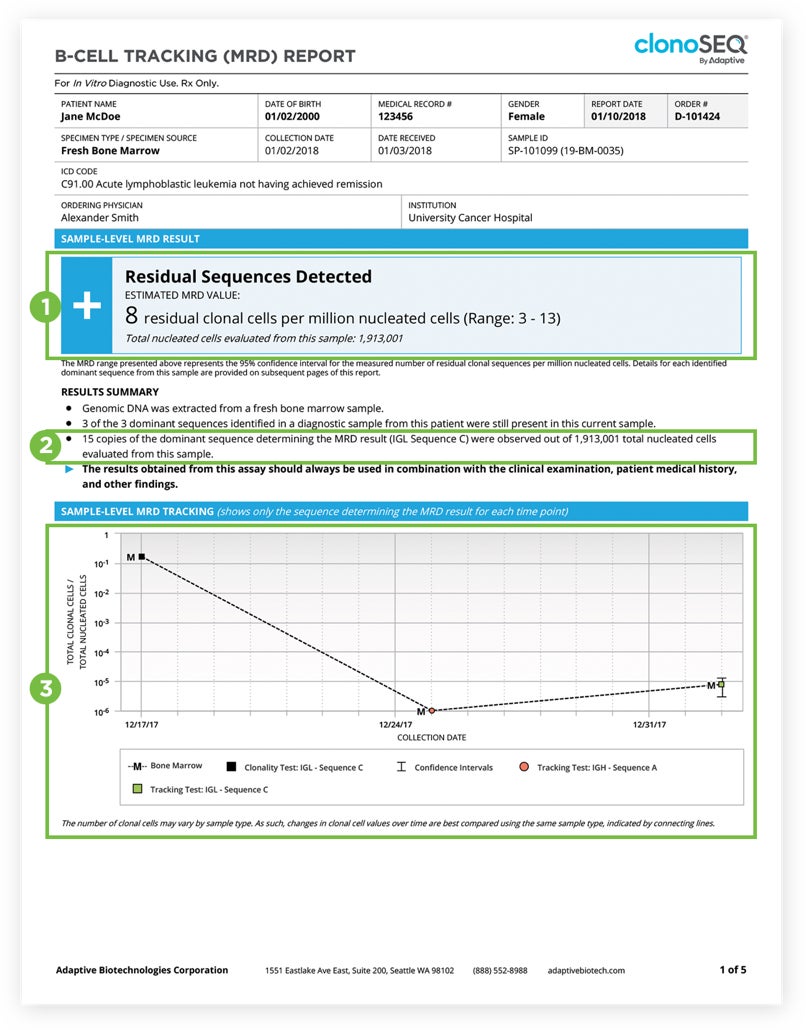
Subsequent reports: MRD report
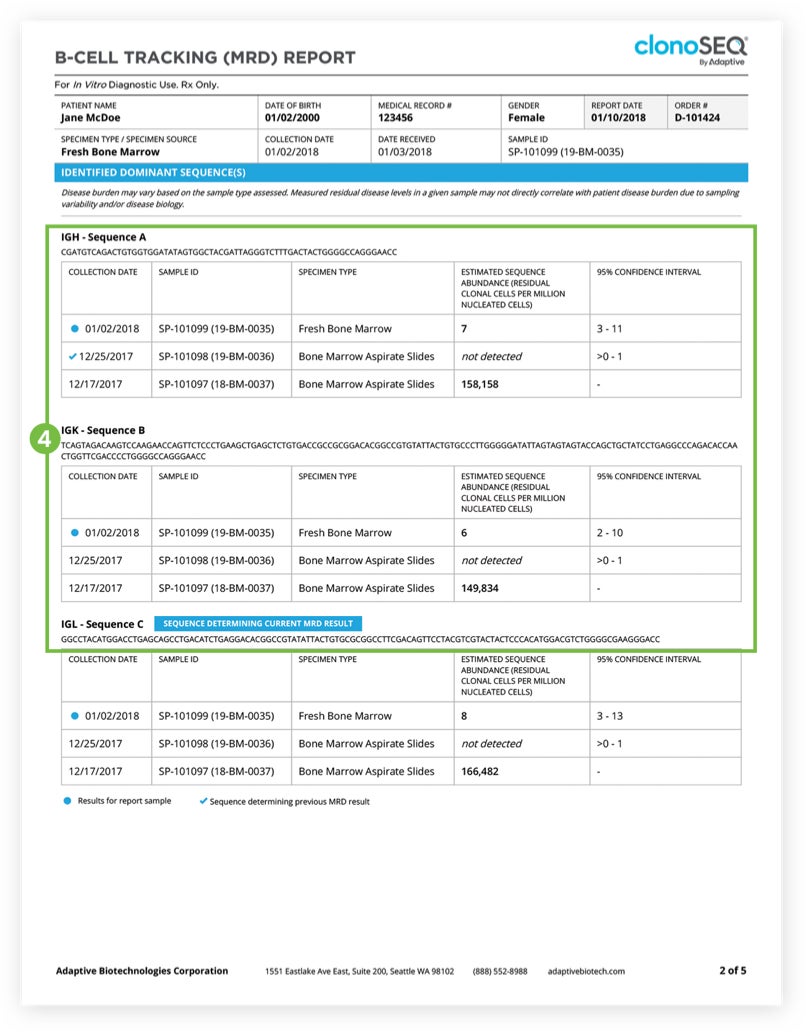
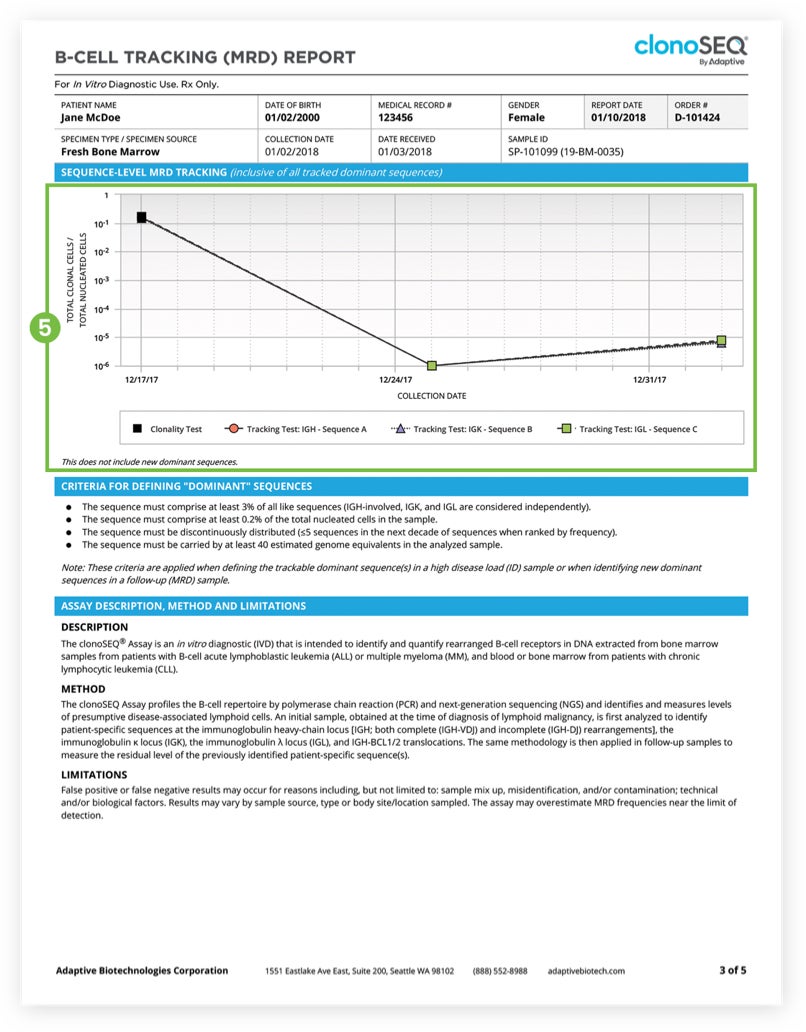
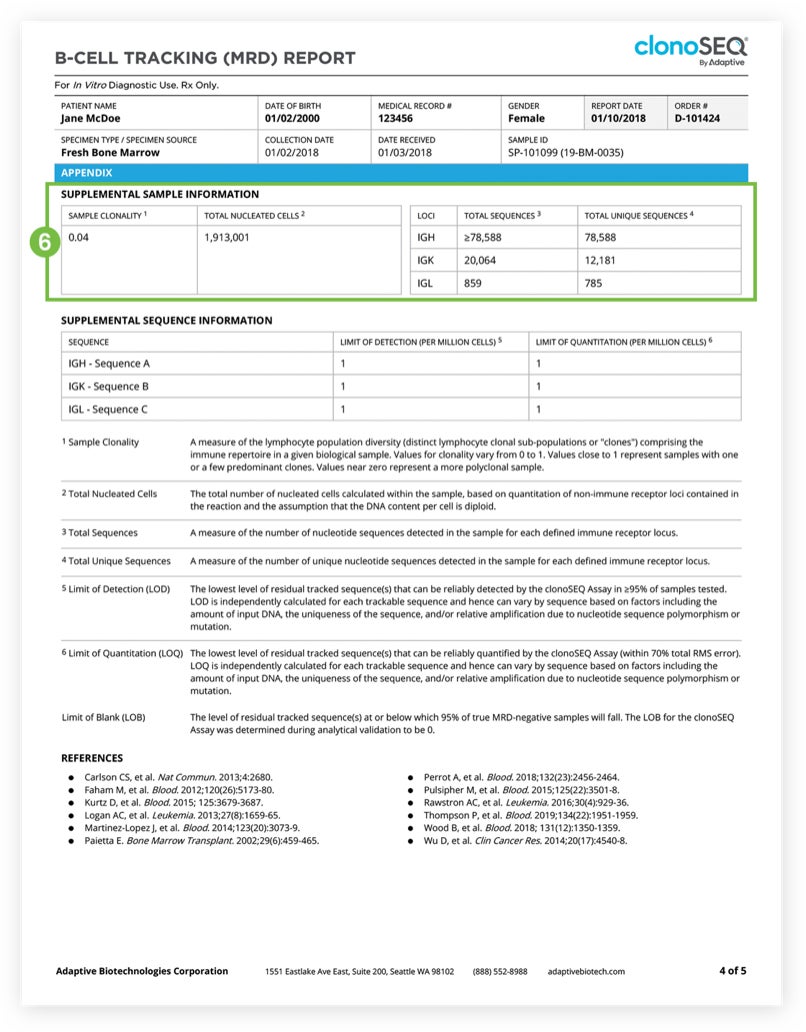
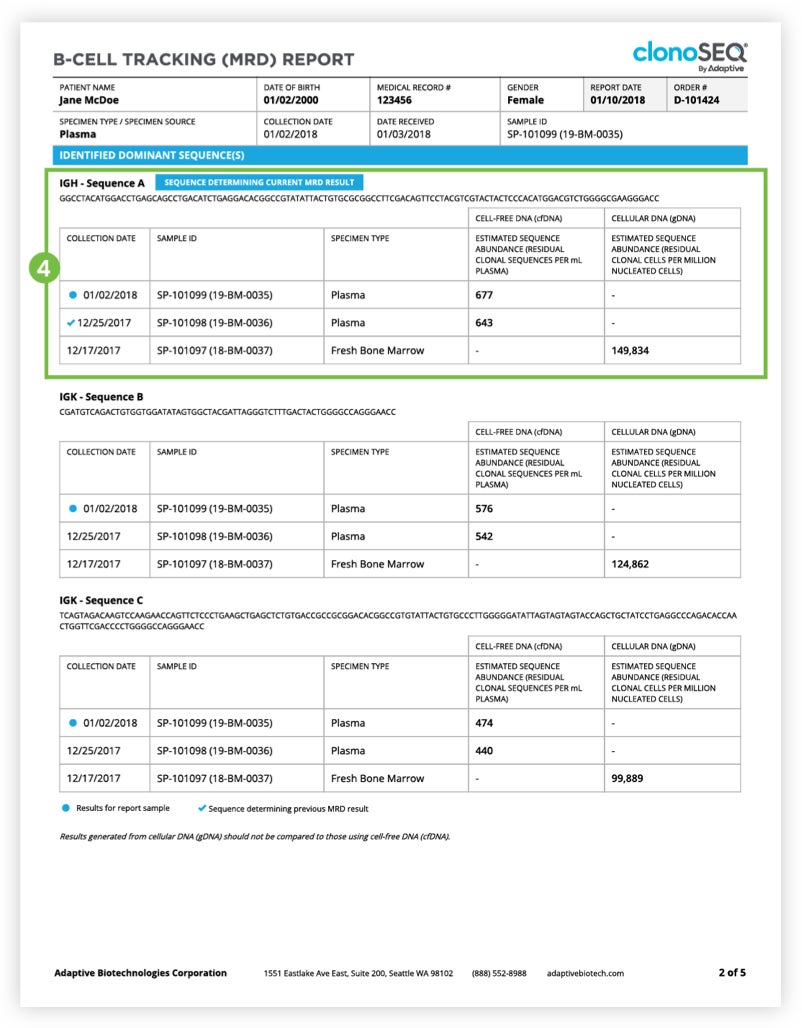
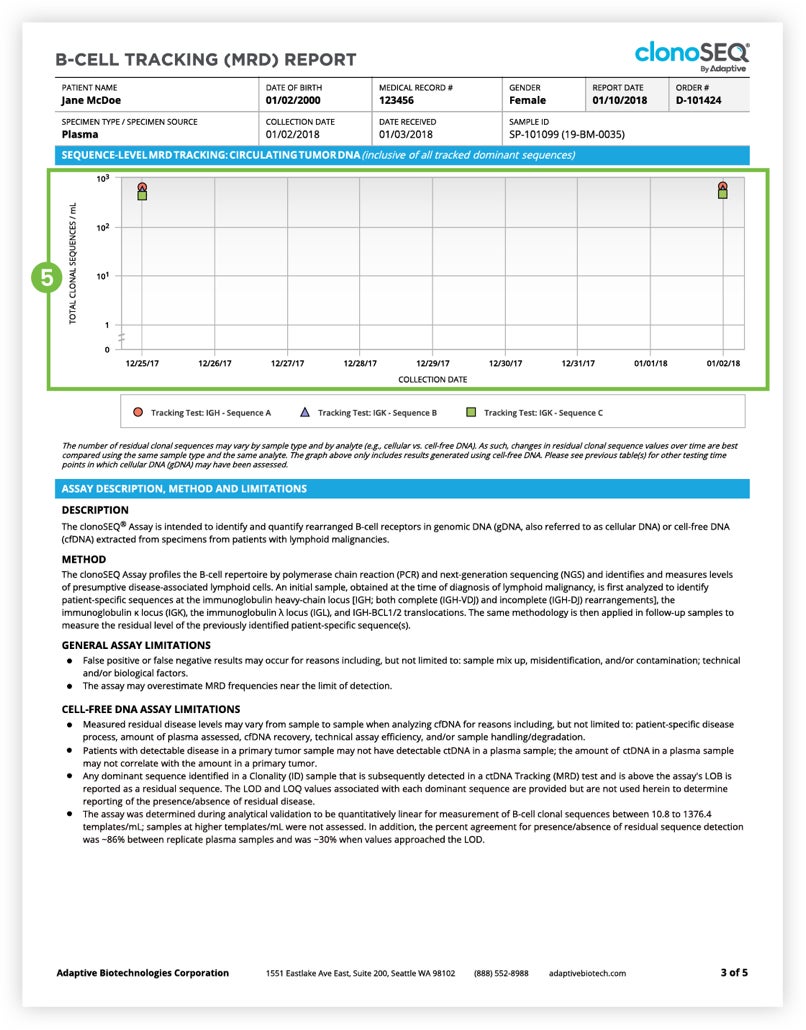
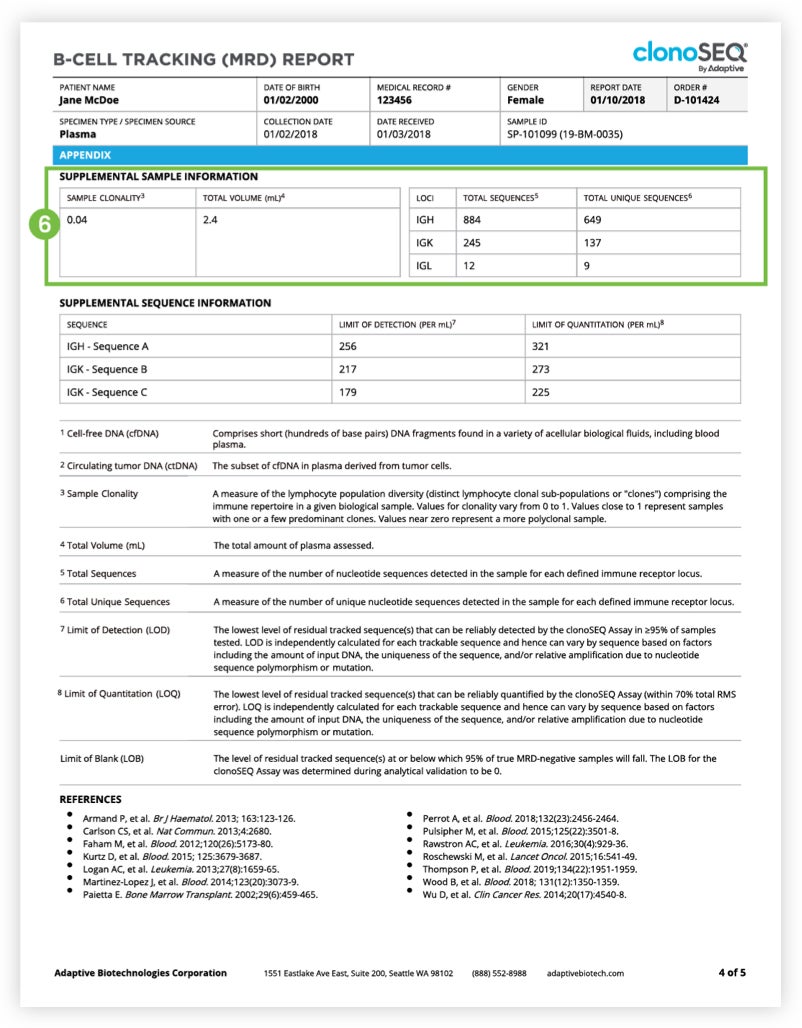
The Tracking (MRD) Report summarizes results from MRD testing of patient samples collected during or after treatment.1 Tracking (MRD) Reports assess and quantify the presence of each tracked DNA sequence and identify newly emerging dominant DNA sequences. Since clinicians often assess residual disease repeatedly during the course of a patient’s treatment or remission, each report provides a visual representation of disease burden over time that clinicians can map to treatment cycles and easily communicate to patients.
The clonoSEQ B-cell Tracking (MRD) Report provides results based on analysis of the IgH, IgK, and IgL loci, as well as Bcl1 and Bcl2 translocations.
MRD tracking report for multiple myeloma, CLL, and ALL
MRD tracking report for DLBCL
This page is intended for a US-based audience.
clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect minimal residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). CLL Clonality (ID) Tests will also produce an IGHV status result, which is provided as a CLIA-validated laboratory developed test (LDT) but which has not been cleared or approved by the FDA. Additionally, clonoSEQ is available for use in other lymphoid cancers and specimen types as a CLIA-validated LDT. For important information about the FDA-cleared uses of clonoSEQ including test limitations, please visit clonoSEQ.com/technical-summary.
Reference
- clonoSEQ®. [technical summary]. Seattle, WA. Adaptive Biotechnologies; 2020.