Diffuse Large B-Cell Lymphoma (DLBCL)
DLBCL
Know where you stand
DLBCL is an aggressive but treatable cancer. The disease starts in B lymphocytes, a type of white blood cell, and usually grows in the lymph nodes. Symptoms include swelling or lumps in the lymph nodes, fever, night sweats, and weight loss.1
Monitoring is important throughout care to measure response to treatment and detect early relapse. Positron emission tomography/computed tomography (PET/CT) imaging is often used, but the results can be inconclusive and this method can’t find cancer cells if there aren’t many of them in the body. That’s why MRD testing can be a helpful disease assessment tool in addition to PET/CT imaging.1-5
When should you monitor with clonoSEQ®?
There can be several stages of treatment for DLBCL. Your doctor may want to monitor MRD at various points to track the state of your prognosis. Your MRD test results with clonoSEQ may guide the choice of which treatment comes next. For example:
Initial diagnosis
Identify cancer cell DNA (Clonality ID Test)
This clonoSEQ test provides a baseline. Because this test will have the largest number of cancer cells, it helps clonoSEQ know which cells to track over time in subsequent MRD tests.
First-line treatment1,6
The most common first treatment for DLBCL is a combination chemotherapy called R-CHOP. Your doctor may want to monitor your progress both during and right after treatment to see how it’s working. You’ll likely get PET/CT scans, but your doctor may also recommend MRD testing.
MRD testing with clonoSEQSurveillance1,6
If you’re in remission and off treatment, your doctor will still likely keep track of your DLBCL for 2 years or more. During this time, MRD testing may be helpful to detect any relapse as early as possible. That will help you plan for potential later-line treatments.
MRD testing with clonoSEQLater-line treatments1,7,8
If you need more treatment, there are a few options. You may get more chemotherapy or immunotherapy, stem cell transplant, or a newer option called CAR T-cell therapy (or a mix of more than one of these). Whatever course you take, MRD testing can help track how well treatment is working and inform next steps.
MRD testing with clonoSEQclonoSEQ in action:
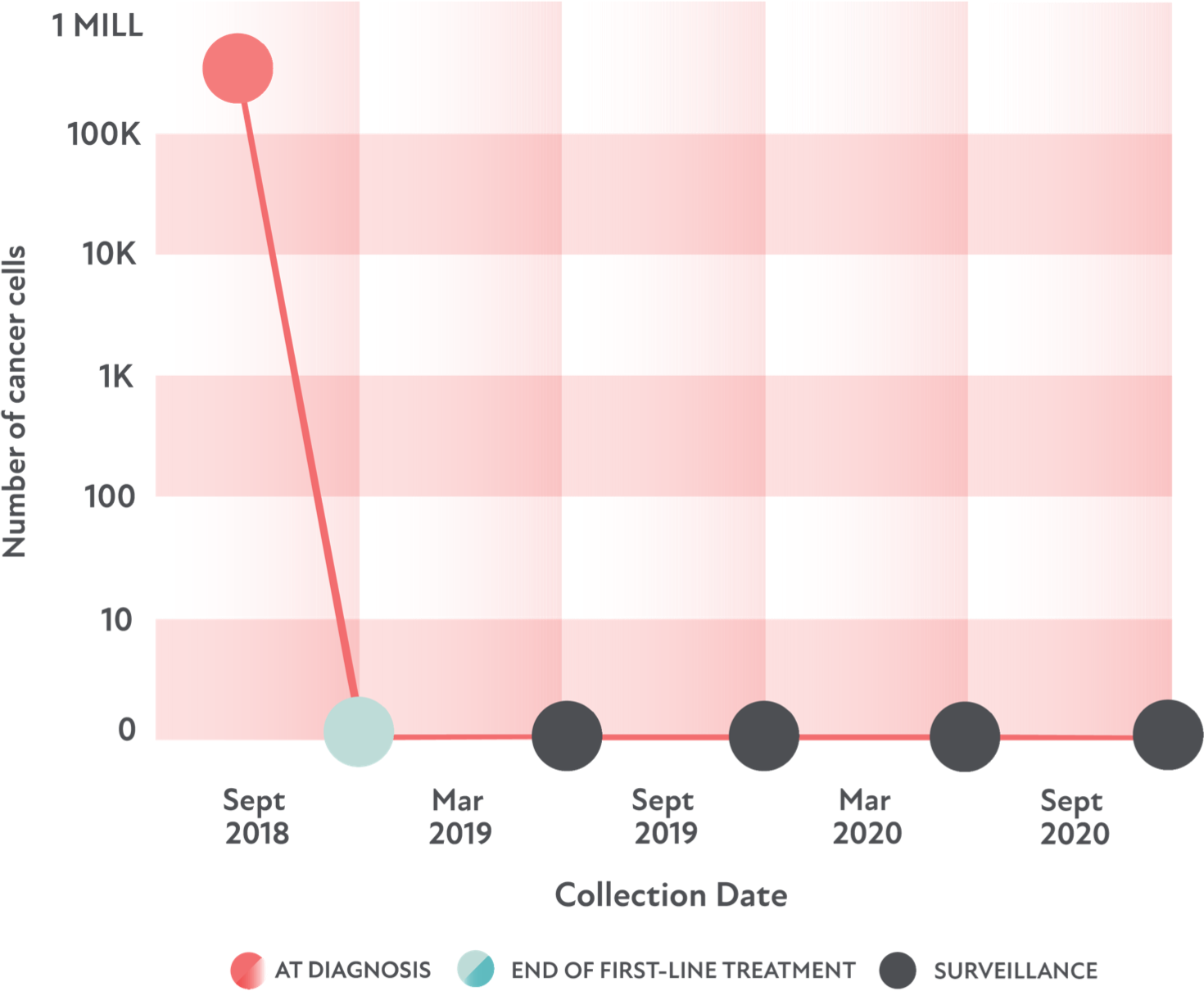
A case study
Let’s look at how clonoSEQ helped a DLBCL patient.
Remember, this is only 1 example—and clonoSEQ has helped people with many DLBCL profiles.
This is for illustration purposes only. The actual clonoSEQ report will look different, as shown here.
- The patient: 65-year-old man
- The goal: To be cured of DLBCL
- The treatment: R-CHOP as first-line treatment
- The result: No cancer cells were detected by MRD or PET/CT imaging after treatment
For 2 years after treatment, the patient received MRD tests every 6 months. They continued to not detect any remaining cancer cells.
If you have DLBCL, talk with your doctor about clonoSEQ. Pinpoint where you are—and come up with a plan that works for you.
Questions to ask your doctor
This page is intended for a US-based audience.
clonoSEQ® is an FDA-cleared test used to detect minimal residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). clonoSEQ is also available for use in other lymphoid cancers and specimen types as a CLIA-validated laboratory developed test (LDT).
clonoSEQ is only available by prescription from a licensed healthcare professional. Results may vary. Talk to your healthcare provider to see if clonoSEQ testing is right for you. For important information about the FDA-cleared uses of clonoSEQ including test limitations, please visit clonoSEQ.com/technical-summary.
References
- Diffuse Large B-Cell Lymphomas. NCCN Guidelines®. Accessed August 31 2022. https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients
- Cohen J, et al. Blood. 2017;129(5):561-564.
- Suh K, et al. Korean J Intern Med. 2019;34(4):894-901.
- Thompson C, et al. J Clin Oncol. 2014;32(31):3506-3512.
- Roschewski M, et al. Lancet Oncol. 2015;16(5):541-549.
- Hu R, et al. Curr Oncol Rep. 2019;21(5):44.
- Merryman RW, et al. Abstract 531 presented at: 62nd ASH Annual Meeting & Exposition; 2020.
- Frank MJ, et al. J Clin Oncol. 2021;39(27):3034-3043.
