Understanding a clonoSEQ® Report
What does your clonoSEQ report mean?
Adaptive Biotechnologies delivers MRD results from your clonoSEQ test to your doctor as a report. Your doctor considers the information in this report along with your physical examination, your medical history, and other test results and findings.
Be sure to talk with your doctor about the optimal timing for MRD testing with clonoSEQ based on the type of blood cancer you have and your specific treatment plan.
References to “cancer” on this webpage refer specifically to multiple myeloma, CLL, B-ALL, and DLBCL. References to “sample” refer to bone marrow or blood from patients with multiple myeloma, B-ALL, or CLL; and bone marrow, blood, or plasma from patients with DLBCL. Talk to your doctor about your options if you have another type of blood cancer and are interested in MRD testing.

clonoSEQ doctor
discussion guide
clonoSEQ is sensitive enough to find a single cancer cell among a million healthy cells, if enough sample material is provided.1*
*clonoSEQ works a bit differently for DLBCL, so the “1 in a million” number does not apply. However, clonoSEQ still detects MRD at a very deep level.
Sample report:
Multiple myeloma, CLL, and B‑ALL
The information on your clonoSEQ report will be individual to you.
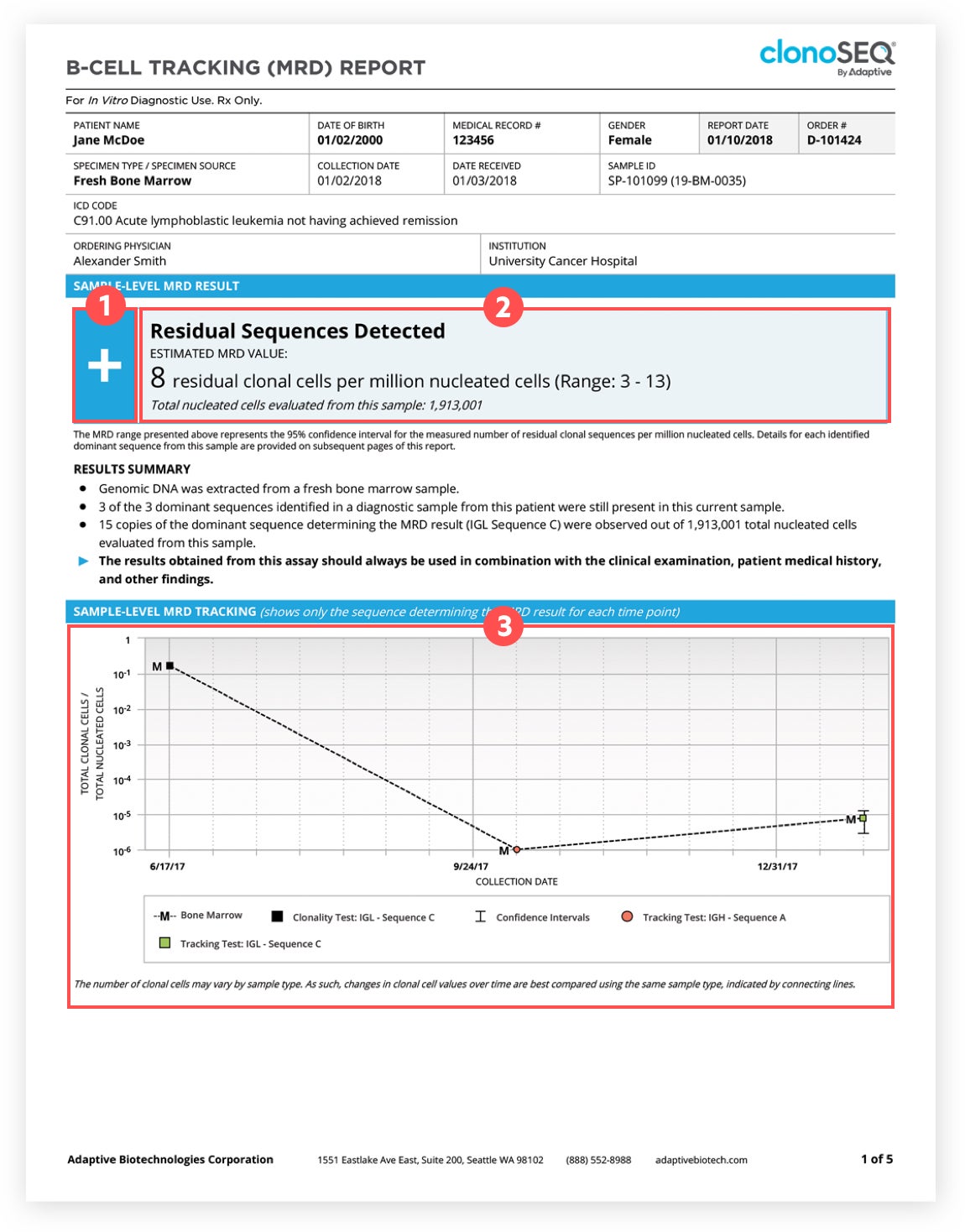
1clonoSEQ MRD Status
A positive (+) result means residual disease was detected. A negative (-) result means residual disease was not detected. Each report will provide your updated MRD status.† You can gain valuable insights about your cancer regardless of whether you have a positive or negative result.
Talk with your doctor about your MRD status to better understand what a positive or negative result means for you and your treatment plan.
†False-positive or false-negative results may occur for reasons including, but not limited to: contamination, technical, and/or biological factors.
2MRD Level
This is the amount of cancer cells detected in your sample. This number shows how much disease is present in your sample when it is taken. Your doctor can help put this number into context based on your current phase of care and treatment goals.
3MRD Trend
A graph will show any changes detected in your MRD level over time. Watching these changes may help you and your doctor better understand your response to treatment and track changes in your levels of cancer cells over time.
Sample report:
DLBCL
The information on your clonoSEQ report will be individual to you.
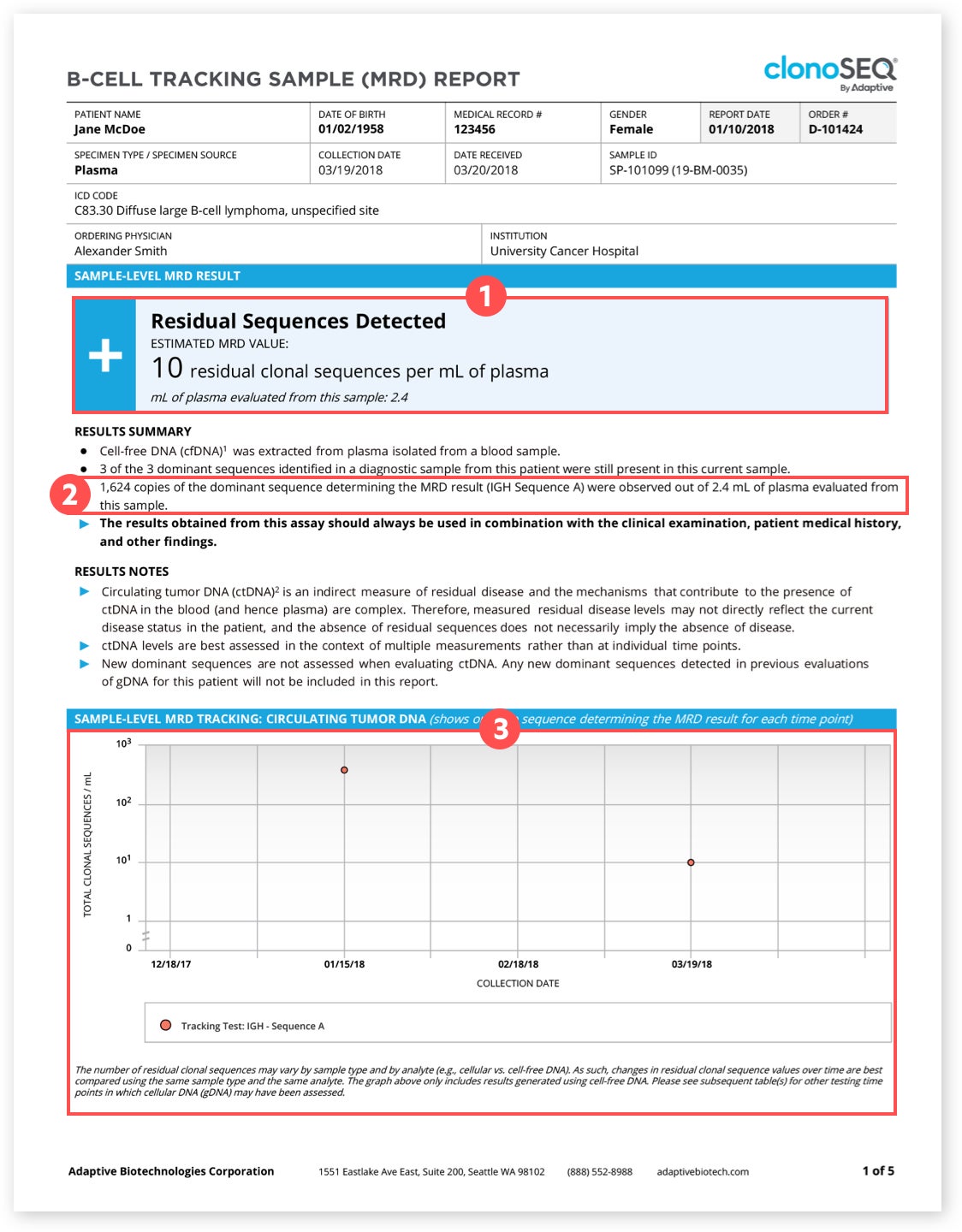
1clonoSEQ MRD Status
A positive (+) result means residual disease was detected. A negative (-) result means residual disease was not detected.‡ The number next to the positive or negative result tells you how many cancer DNA sequences were found when a mL of the plasma from your blood was separated and analyzed.
Talk with your doctor about your MRD status to better understand what a positive or negative result means for you and your treatment plan.
‡False-positive or false-negative results may occur for reasons including, but not limited to: contamination, technical, and/or biological factors.
2Total MRD Level
This is the total amount of cancer cells detected in your sample. Because these cells divide over time, the total MRD level will always be greater than the number of sequences detected in the box above.
3Sample Level Results Over Time
This graph will show any changes in your MRD level over time. These are “sample level” results, meaning that the cancer DNA sequence that was seen the most in your sample is the one plotted on the graph.
Watching these changes may help you and your doctor better understand your response to treatment and track changes in your cancer cells over time.
This page is intended for a US-based audience.
clonoSEQ® is an FDA-cleared test used to detect minimal residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). clonoSEQ is also available for use in other lymphoid cancers and specimen types as a CLIA-validated laboratory developed test (LDT).
clonoSEQ is only available by prescription from a licensed healthcare professional. Results may vary. Talk to your healthcare provider to see if clonoSEQ testing is right for you. For important information about the FDA-cleared uses of clonoSEQ including test limitations, please visit clonoSEQ.com/technical-summary.
Reference
- clonoSEQ®. [technical summary]. Seattle, WA: Adaptive Biotechnologies; 2020. https://www.clonoseq.com/technical-summary
